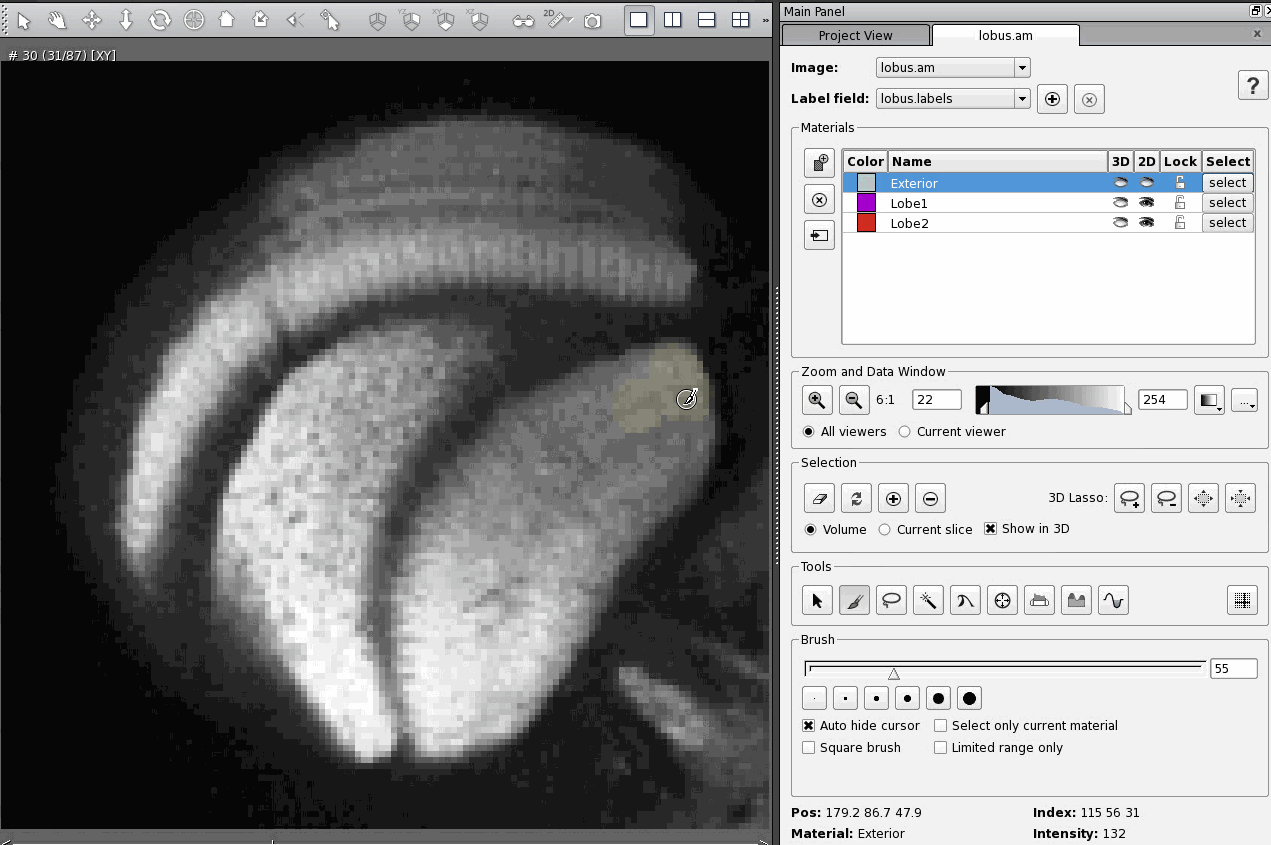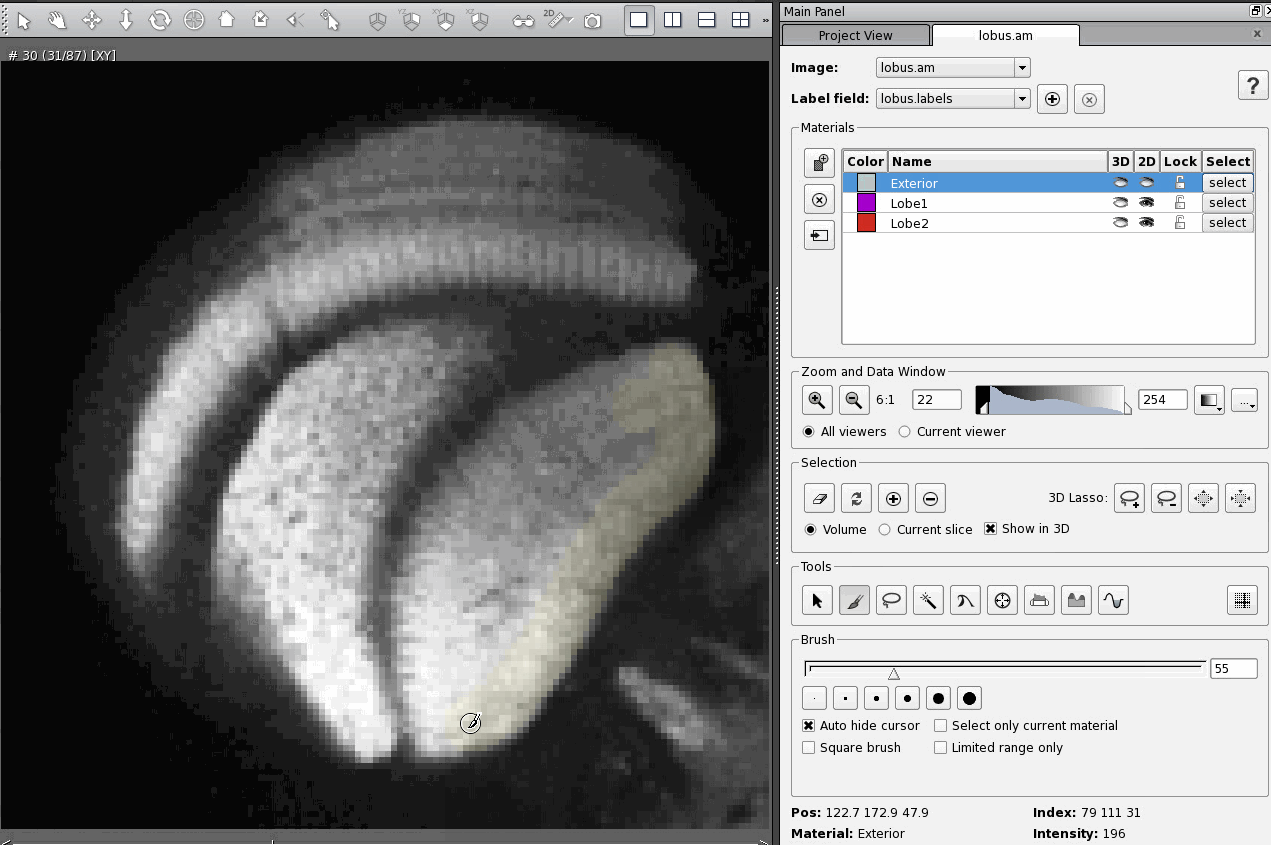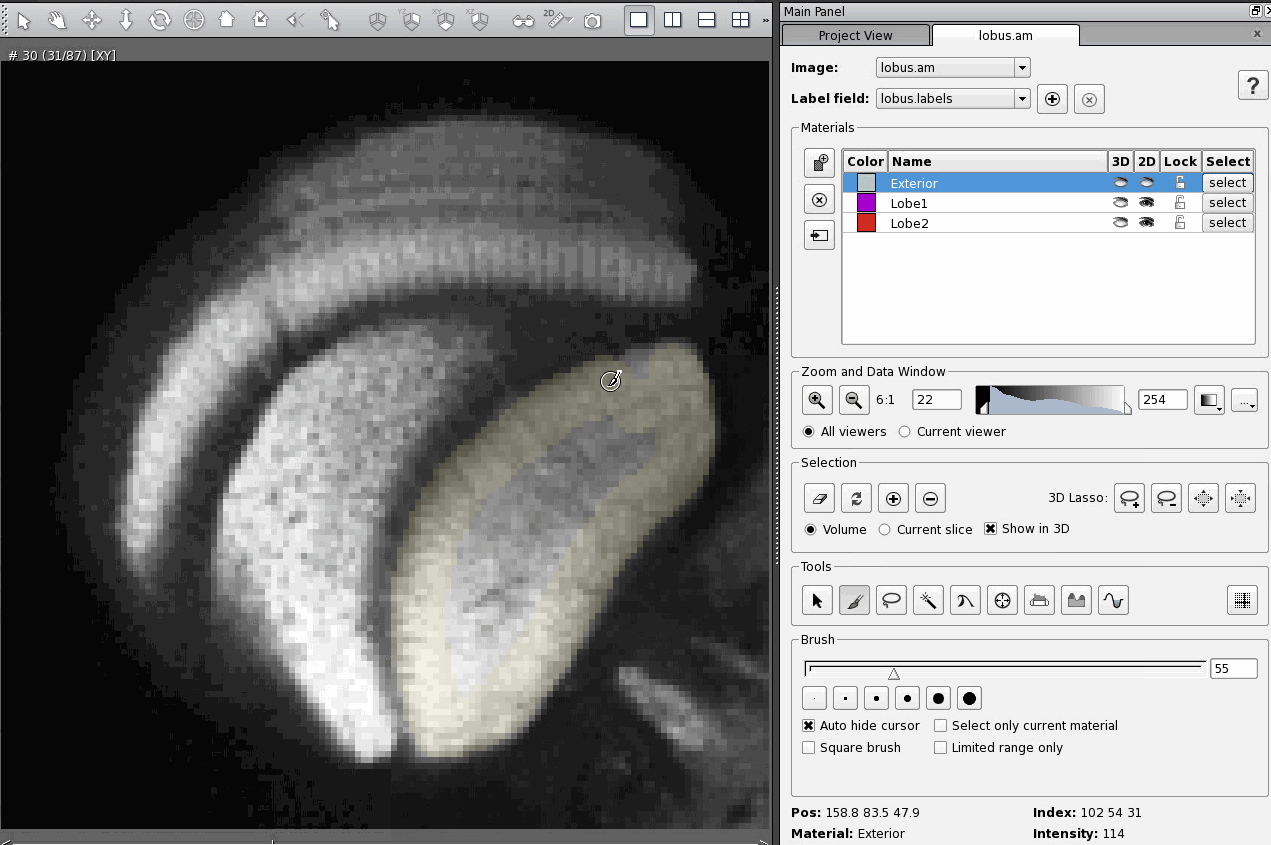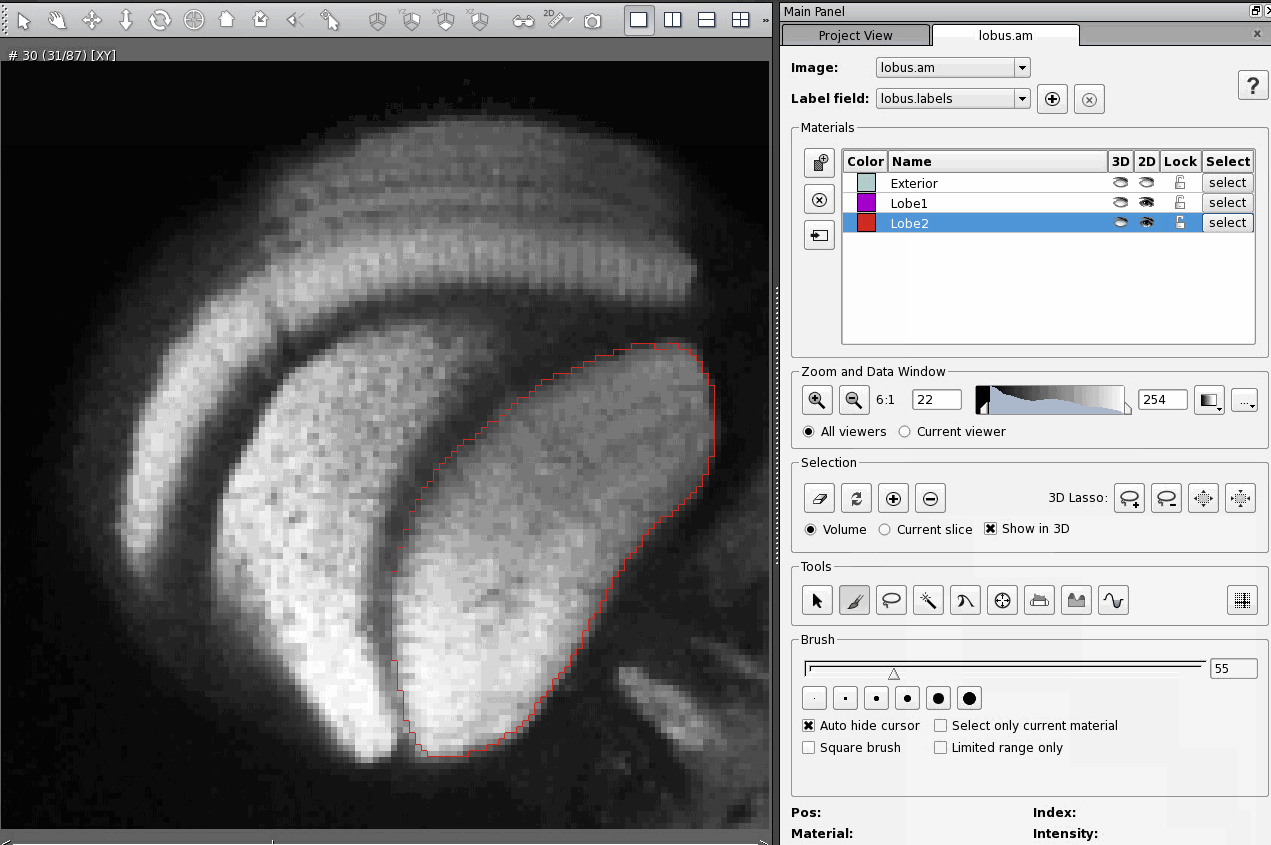
A day after posting my response to an email asking about CT scanning, I received a fresh question in my inbox asking about quite a different topic: immunohistochemistry using antibodies against the protein Netrin-1.
De : [redacted]
Envoyé : 25 janvier 2023 17:27
À : [redacted, forwarded to me]
Cc : [redacted]
Objet : advice on NTN1 ab
I am writing to seek advice regarding the choice of antibody (and method) for fluorescence immunocytochemistry against Netrin-1 in rat cultured [redacted] neurons. My student found your paper on optimizing immunohistochemistry with the Novus and Abcam Ab https://pubmed.ncbi.nlm.nih.gov/29276694/, and he also found this antibody: https://www.lsbio.com/pathplus-antibodies/pathplus-ntn1-antibody-netrin-1-antibody-aa591-604-if-immunofluorescence-ihc-ls-b5912/141319
Do you still use these antibodies, and if so which one would you suggest for our application? We weren’t sure whether citrate, SDS and/or heat would do well in dissociated neurons, so we haven’t yet tried. When [my student] tried the [abcam Netrin-1 antibody] without any specific treatment (just PFA +/- triton X), he found fairly weak staining (but does detect over-expressed GFP-tagged NTN1).
What do you think ?
Here’s my response, which probably goes into more detail than the inquirers really needed, but hopefully has some useful information for them (and anyone else with similar questions):
In my experience the abcam antibody is more sensitive than the NOVUS one, so I think you’re on the right track using the abcam one. First, a disclaimer that I’ve only ever done immuno optimization on fixed tissue, never on cultured cells, but my understanding is that, in general, it’s easier to get a signal from cultured cells than from tissue. With that being said:
Is the faint signal you’re currently getting sufficient for your purposes? Often I can turn a faint signal into something useable (for data collection, taking images for publication, etc) by fiddling with the microscope settings, in particular illumination intensity, light strength, and gain (but be careful of bleaching!) Also, signal gets stronger with increasing magnification; often I find that a signal that’s barely discernable at 5X magnification looks quite nice at 40X or 100X. Can you do your work at higher magnification?
If none of that works for you, there are umpteenth variations (and combinations of variations) of immunohistochemistry protocols that you can try. The simplest that I’ve had some success with are: leaving the samples to incubate in the primary antibody for longer (so try for 3 days if you were incubating for 2, for example), doing your incubations on a rocker, adding the serum of the animal your secondary is from to your blocking solution (but I think this is more to reduce background than to increase signal), increasing the concentration of triton-X (or tween-20, whichever you are using) in your blocking solution, and using the commercial Protein Block and Antibody Diluent solutions from Agilent instead of home-made blocking solution. In all honesty I don’t think any of these would work to improve Netrin-1 staining in fixed tissue, but because - I think - staining cultured cells is easier, they might be worth a try for you.
If you want to try antigen retrieval methods, be warned that these do tend to make samples more fragile, and I would try them one at a time before trying them in combination. The mildest one I can think of is EDTA, a calcium chelating agent. My recollection is that we never tried EDTA to recover Netrin-1 signal because we had citrate and SDS in the lab but didn’t have EDTA. Citrate is the next mildest, and SDS is the harshest. So I would try Citrate before SDS, and I would try each individually before trying them in combination.
Something I didn’t mention in the email but thought of after is that I should have sent an example protocol for antigen retrieval using EDTA, since I don’t have any personal experience with it and it’s not included in my Netrin-1 immunohistochemistry methods paper (which the inquirer linked to in their email). Here is the paper I’m familiar with that talks about using EDTA for antigen retrieval: Possible role of tissue-bound calcium ions in citrate-mediated high-temperature antigen retrieval - PubMed (nih.gov).
If you have any comments, advice, or corrections on the advice I’ve given here, I’d love to hear from you, so please get in touch!