I've spent a good portion of my PhD working with 3D images in the program Avizo, which is essentially the same as the program Amira. This involved a steep learning curve and a lot of problem solving in the beginning. I've decided to write about the problems I've encountered and how I dealt with them in the hope that this may help the next person learning to use Avizo or Amira do so a bit faster, and with less frustration along the way. These problems will look trivial to the regular or advanced Amira/Avizo user, but they were quite frustrating at the time! These posts assume the reader's familiar with sections 2.1-2.5 of the Amira user's guide, which cover how to load, view and segment an image. My introductory post on this topic is here.
In this post I'm going to talk about some additional issues with the hot keys and with adding and subtracting to materials in the Image Segmentation Editor.
An additional hot key.
Here is a list I downloaded of the hot keys that you can use with the Image Segmentation Editor to really speed up segmentation. I've discussed them previously. There's one omission to the list (that I've found, anyway). Keys 1-9 cover the different tools, starting with the Brush tool, but what about the Pick & Move tool? Turns out, even though it's not on the list, "0" switches ("toggles") you to the Pick & Move tool! I've updated my list accordingly.
The add and subtract hot keys are finicky
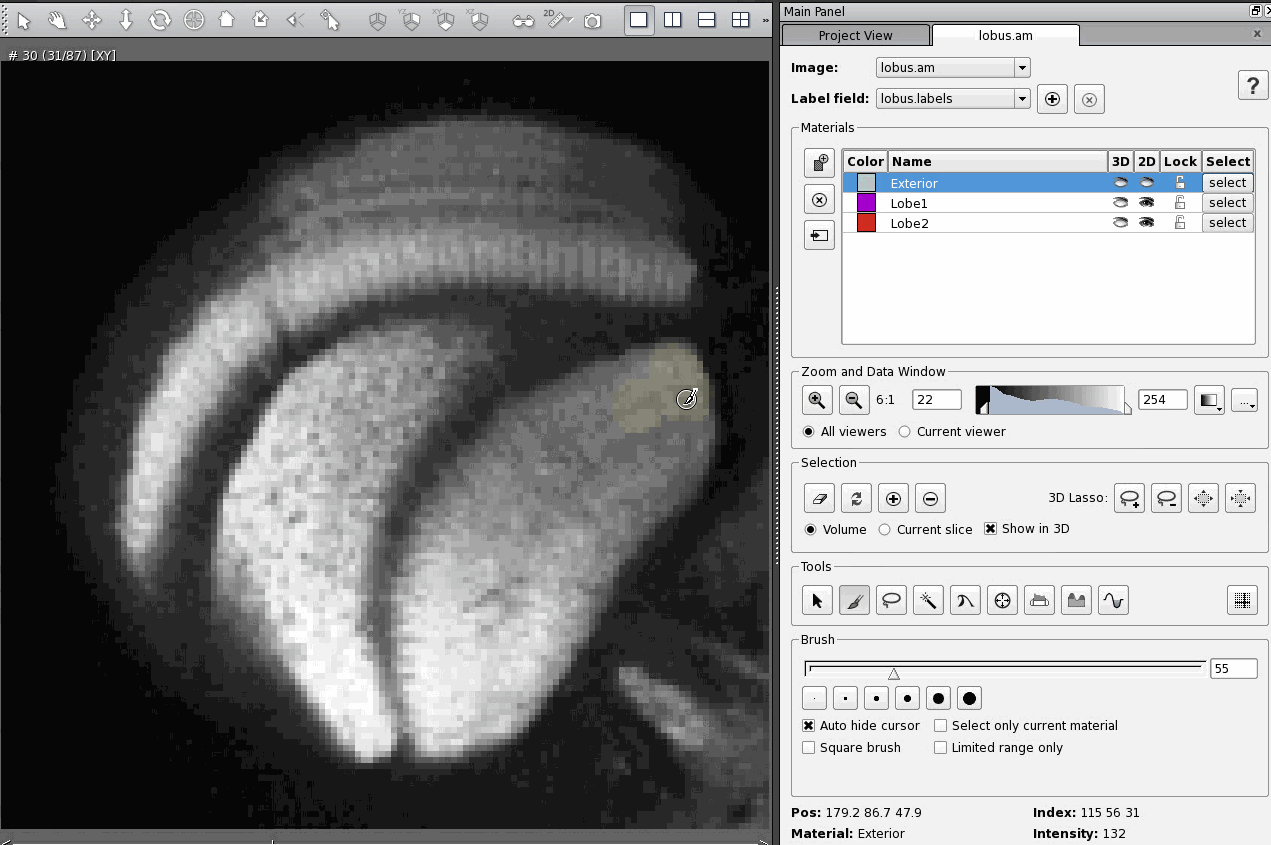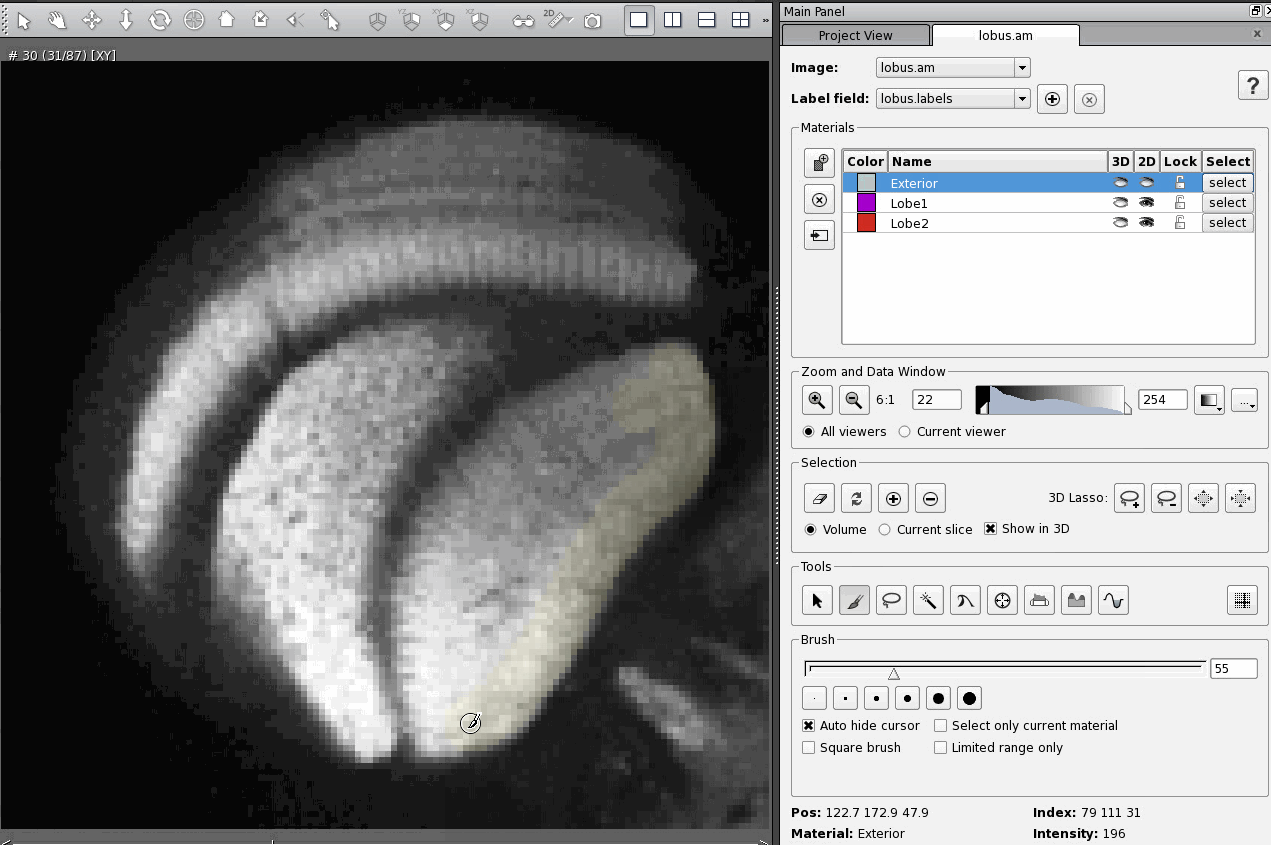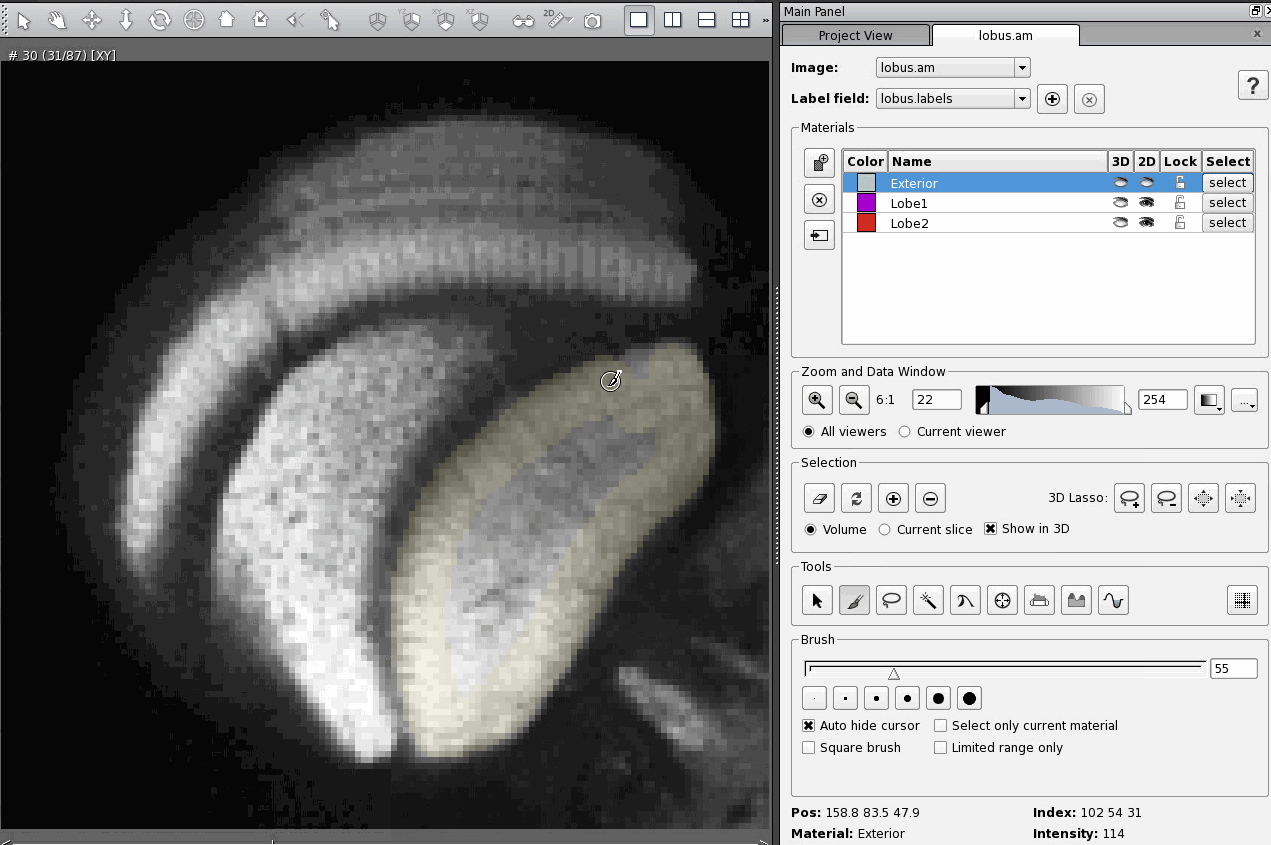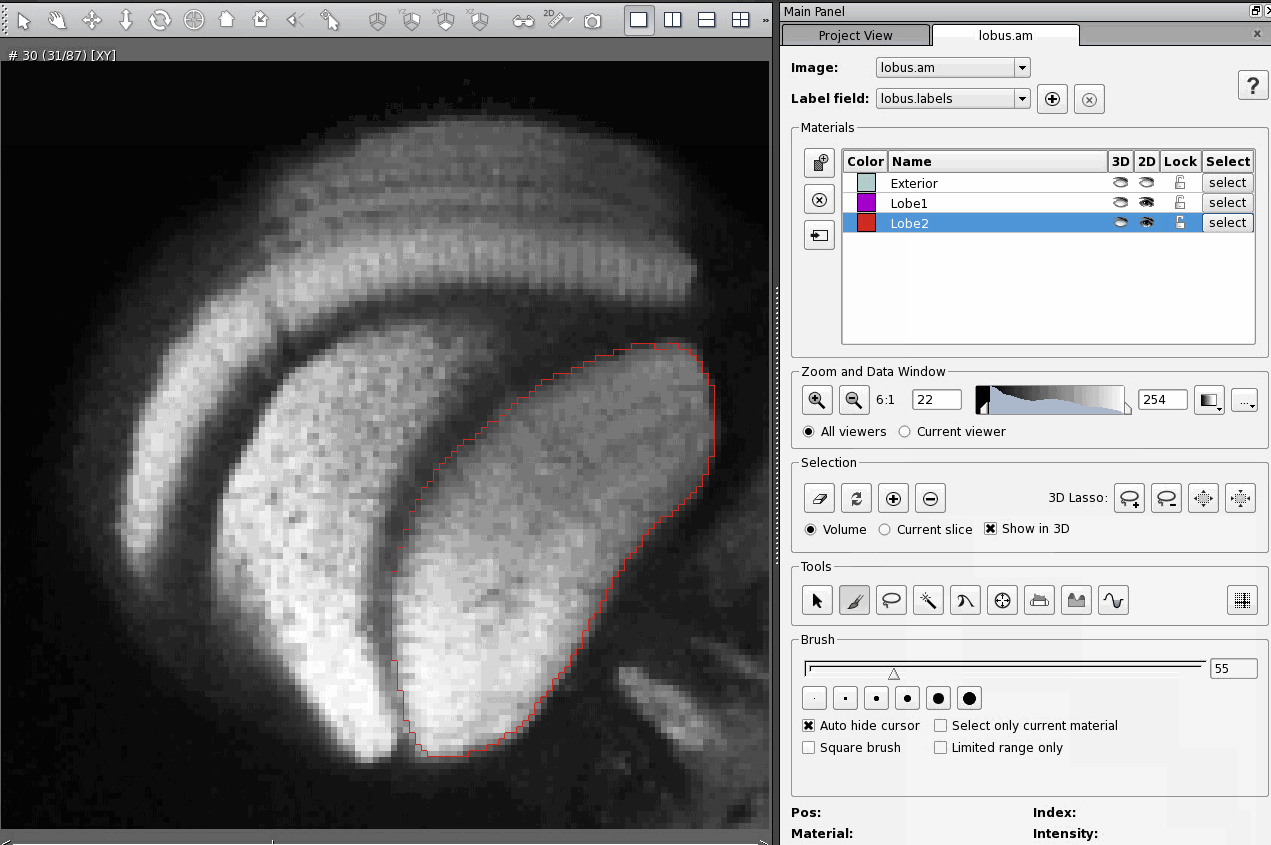
The most straightforward and least efficient way to add a region of an image to a material is to select that region using whichever tool is most appropriate (in these examples I use the brush tool, which is the most straightforward tool, but again the least efficient) and then click on the Add button in the Selection section of the Segmentation Editor. Here's what that looks like:
There are two hot keys that are supposed to do the same thing, "A" and "Ctrl +". The trick is, neither works in exactly the same way. Once I figured out the differences between them it was quite useful, because now I can use whichever Add function best suits my needs at the time. But it was confusing to figure out.
Pressing "Ctrl +" adds the selected part of the image to the material, but leaves it selected. You must then remember to unselect the region manually using the Clear button in the Selection section of the Segmentation Editor.
That means that, if you then select a region that you want to add to a different material, say Lobe1, and then go to add your new selection to Lobe1, you will add both selections to Lobe1 unless you remember to unselect your first selection.
Using the "A" key to add a selection to a material is even more tricky. It only works if you've already added at least one selection to the material since editing any other material. So, practically, the "A" key will only add a selection to the material if the last thing you did was add (or subtract) a selection to the same material. Furthermore, where that previous selection was matters, and depends on your current settings. In the Selection section of the Segmentation Editor, if the button "Volume" is selected, then the previous selection you added could have been on any slice.
However, if the button "Current Slice" is selected in the Selection section of the Segmentation Editor, then the previous selection added to the current material (in this case, Lobe2) must have been in the current slice for the A hot key to work.
The "-" hot key for subtracting a selection from the current material has the same sort of funny restrictions as the "A" hot key.
If you have any suggestions, feedback, or questions about Amira/Avizo, please let me know.